Question 3

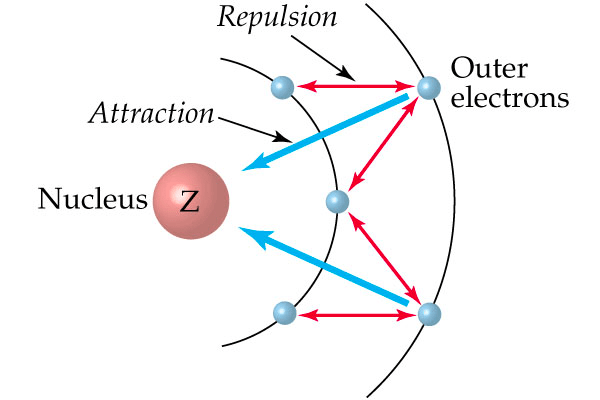
Question 9
- In an electrochemical reaction, as the reaction progress, the voltage of the cell will decrease because with a lower concentration, the number of electrons that are being transferred will decrease
Question 12
SF4 has an asymmetrical molecular structure
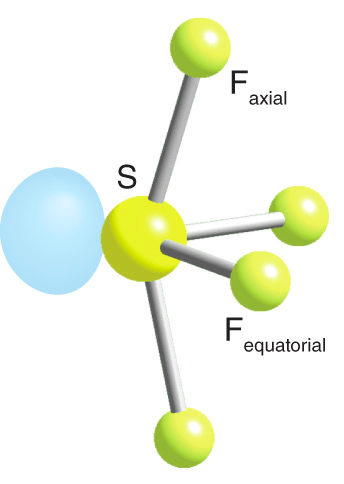
Question 16
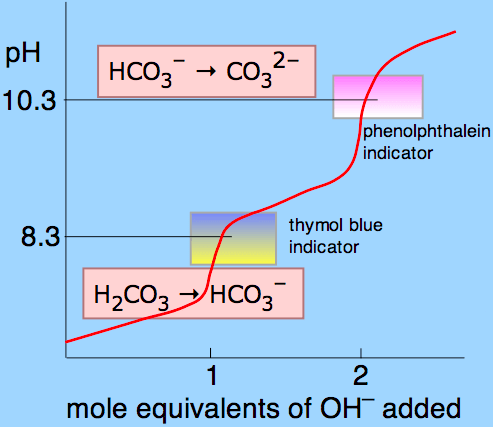
Question 17
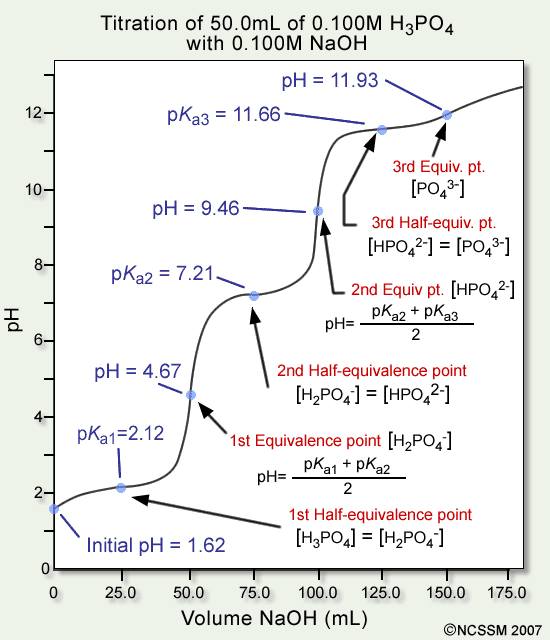
![计算机生成了可选文字: Henderson-Hasselbalch Equation \[A \] pH = pKa + 《 og
\[HAJ \[HA\] ConJ ugate Base Weak Acid](media/image272.png)
- At the half-equivalence point, the last part of the Henderson-Hasselbalch equation cancels out, leaving pH=pKa
Question 20
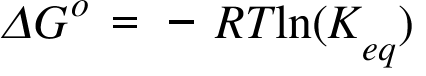
Question 21

Question 23
- Buffer = a week acid/base + its salt
Question 27
- With the same amount of limiting reactant, adding more excessive reactant will not change the value for H, but the change in temperature will decrease for the increase in overall mass
Question 38
- At low temperatures or high pressures, real gases deviate significantly from ideal gas behavior.
Question 49
- The overall rate law is always equal to the rate law for the slowest elementary step, which can be determined using the coefficients of the reactants. In this case, rate = k[NO2][F2]. To get the overall order, we add the exponents in the rate law. 1+1 = 2
Question 53
Lower vapor pressure = weaker IMFs
IMFs:
polar > nonpolar
with hydrogen bonding > without hydrogen bonding
Question 55
- If both nonpolar, more electron = more polarizable = stronger IMFs = higher boiling point
Question 60
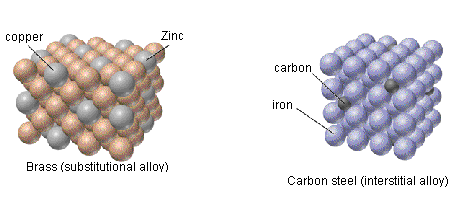
- Substitutional alloys have similar, reduced malleability and ductility to interstitial alloys and have densities that typically lay between the densities of the component metals