Question 16
Question 21
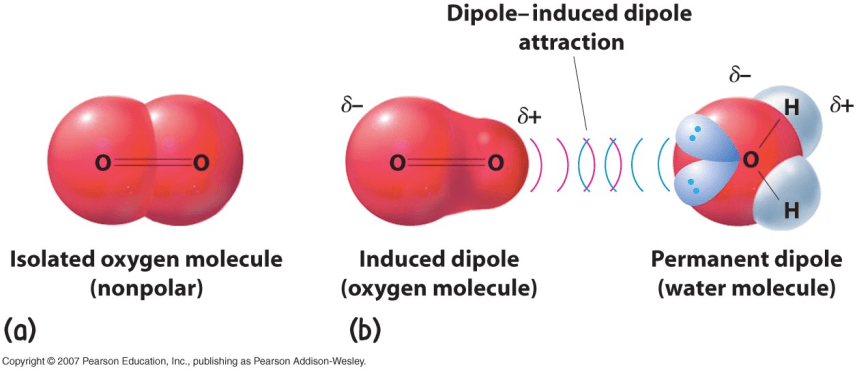
Dipole-induced dipole interaction
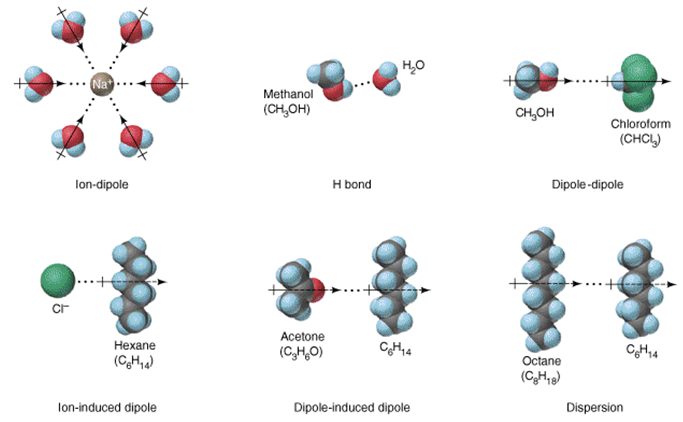
Question 29
ΔH° = bond enthalpies for the bonds broken - bond enthalpies for the bonds formed
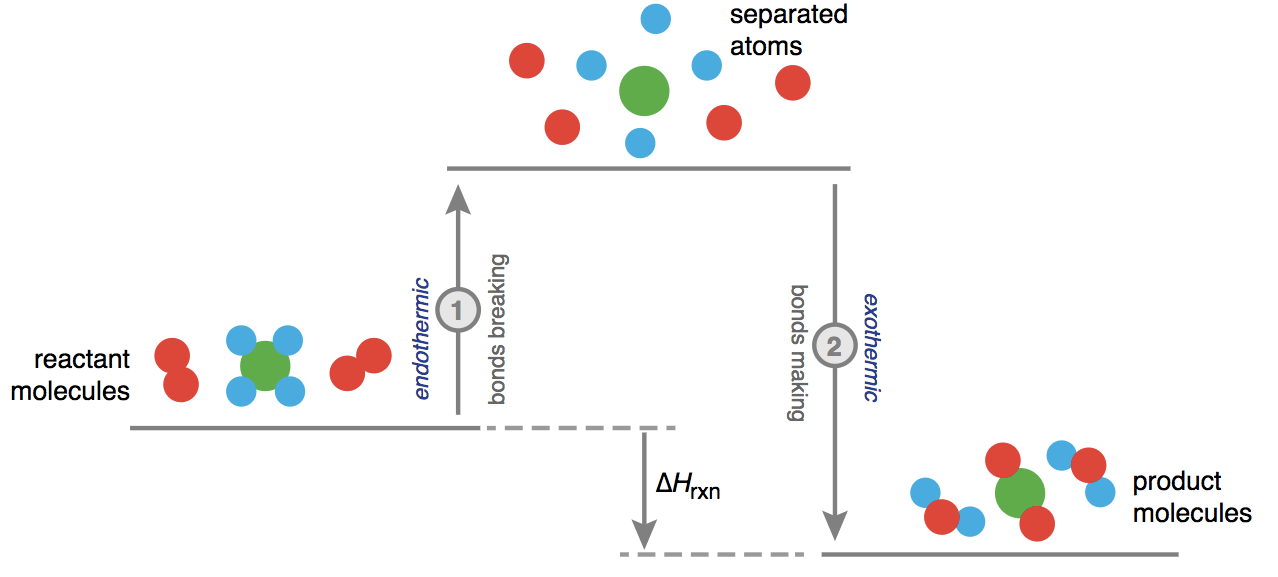
Question 34

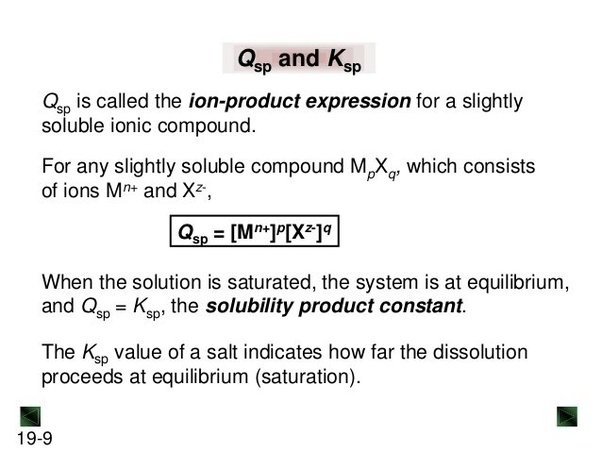
Question 43
Intermediate should not be lower than products
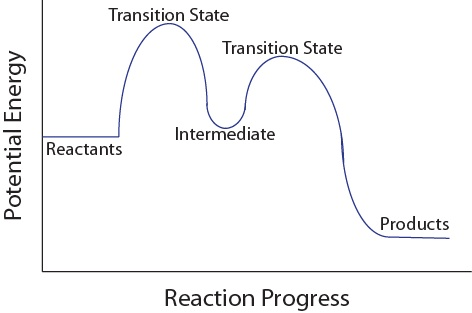
Question 49
Chromatography Experiment
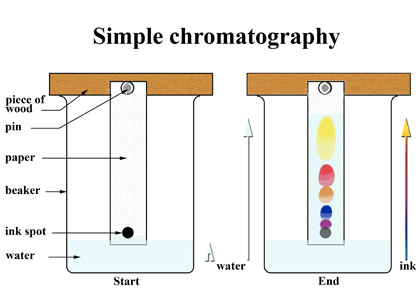
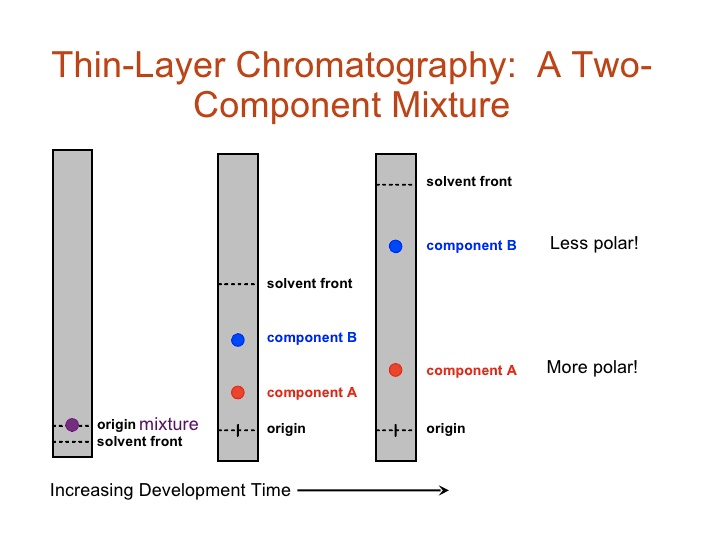

Question 50
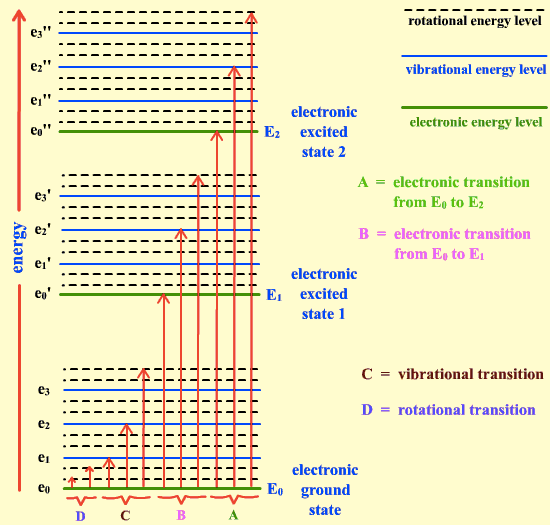
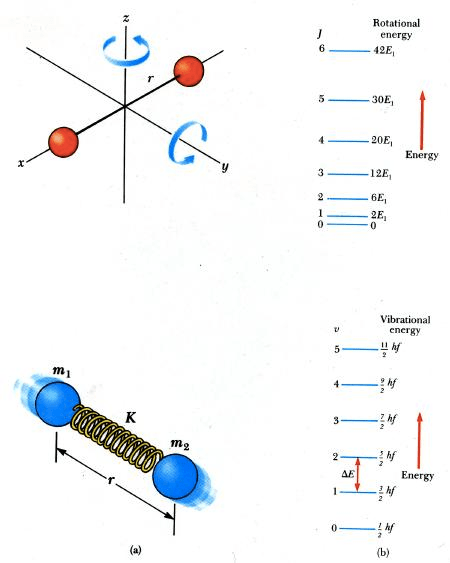
Electronic transition < Molecular vibration < Molecular rotation