Question 1 (a)
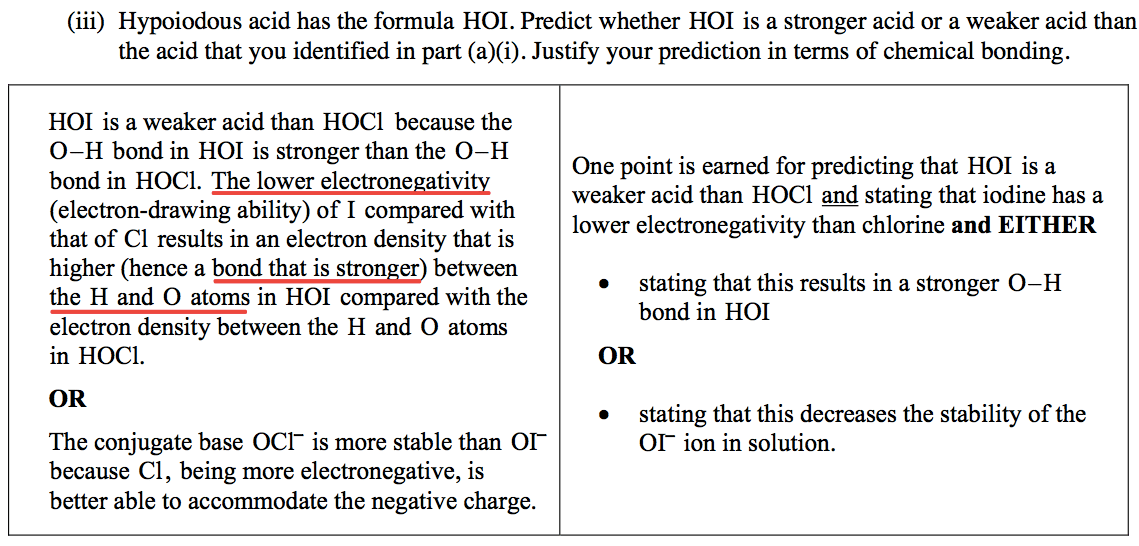
HOI is a weaker acid than HOCl
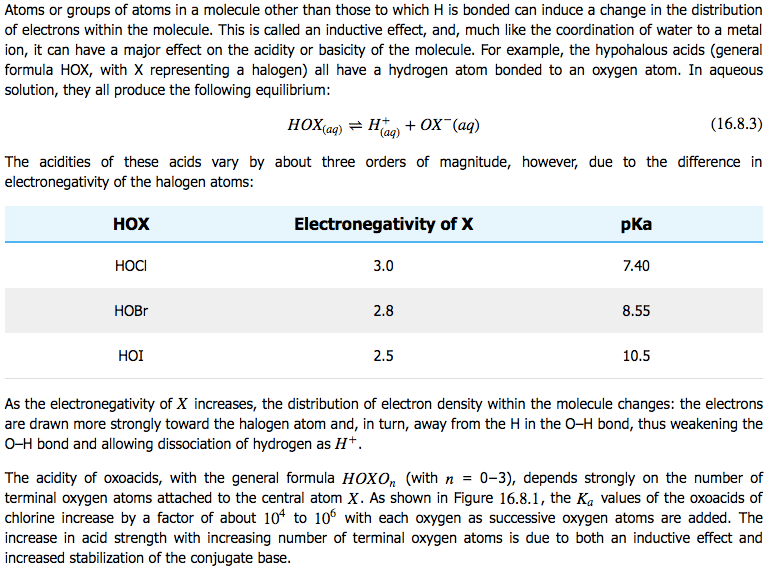
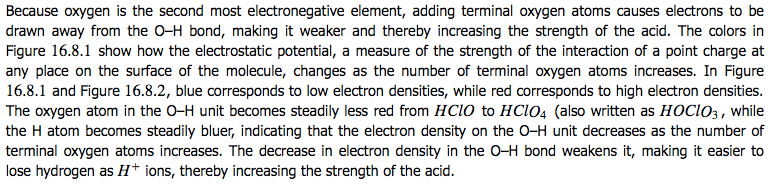
Inductive Effects
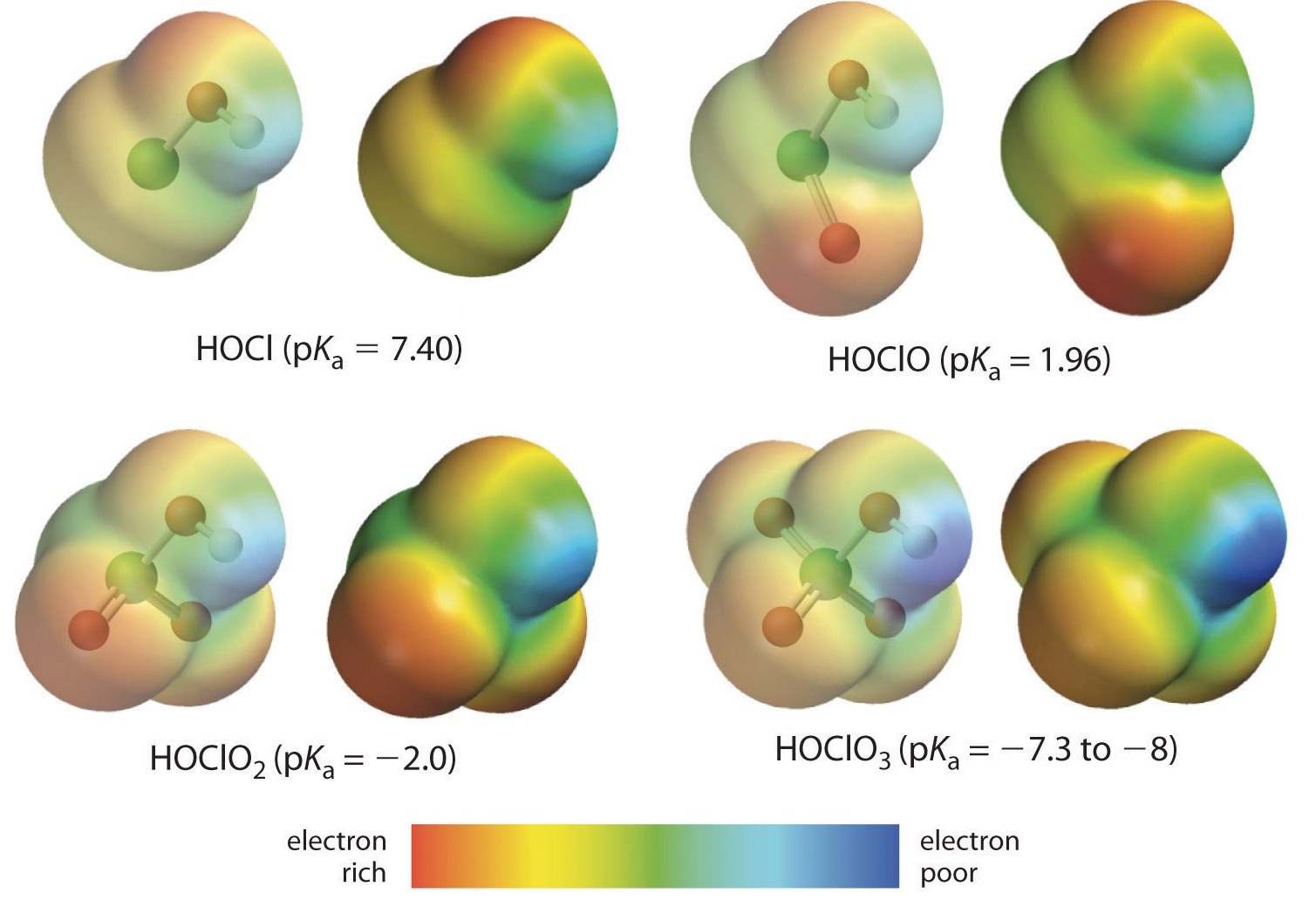
Question 2 (e)
Percent error is always positive
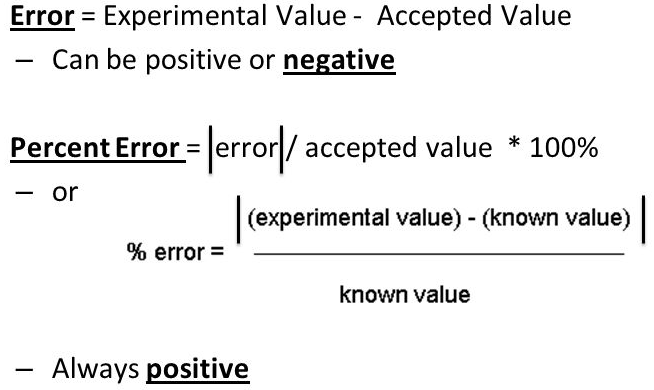
Question 3 (e)
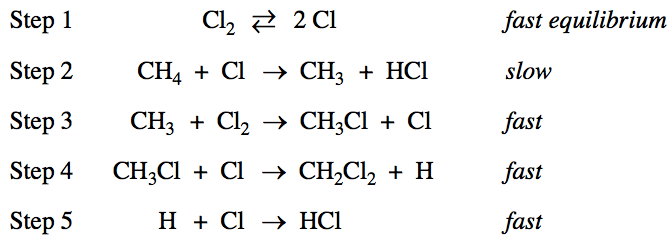
![1 The order of the reaction with respect to C12 is \[Cl\]2 -z.
\[Cl\] 1<1/2 \[Cl \]1/2 For step 1, K - \[C121 Substituting into the
rate law for step 2 (the slowest step in the mechanism): rate-
k\[CH4\] \[Cl\] \[C12\]l/2) \[CH4\] \[C12\]l/2 Because the exponent of
C12 in the rate law is 1/2, the order of the reaction with respect to
C12 is 1/2. One point is earned for the correct answer with
appropriate justification.](media/image87.png)
Mechanisms with a Fast Initial Step
![Let's consider an alternative mechanism that does not involve a
termolecular step: (fast) NO(g) + Bh(g) Step 1: step 2: NOBh(g) +
NO(g) 2NOBr(g) (slow) \[14.27) In this mechanism, step 1 involves two
processes: a forward reaction and its reverse. Because step 2 is the
rate-determining step, the rate law for that step governs the rate of
the overall reaction: Rate = \[14.28) Note that NOBr, is an
intermediate generated in the forward reaction of step 1. Inter-
mediates are usually unstable and have a low, unknown concentration.
Thus, the rate law of Equation 14.28 depends on the unknown
concentration of an intermediate, which isn't desirable. We want
instead to express the rate law for a reaction in tern-IS of the
reactants, or the products if necessary, of the reaction. With the aid
of some assumptions, we can express the concentration of the inter-
mediate NOBr2 in terms of the concentrations of the starting reactants
NO and Br2. We first assume that NOBr, is unstable and does not
accumulate to any significant extent in the reaction mixture. Once
formed, NOBr2 can be consumed either by reacting with NO to form NOBr
or by falling back apart into NO and Br,. The first of these
possibili- ties is step 2 of our alternative mechanism, a slow
process. The second is the reverse of step 1, a unimolecular process:
NO(g) + NOBr2(g) Because step 2 is slow, we assume that most of the
NOBr2 falls apart according to this reaction. Thus, we have both the
forward and reverse reactions of step 1 occurring much faster than
step 2. Because they occur rapidly relative to step 2, the forward and
reverse reactions of step 1 establish an equilibrium. As in any other
dynamic equilib- rium, the rate of the forward reaction equals that of
the reverse reaction: kCNO\]CBr2\] Rate Of forward reaction CNOBr2\]
Rate Of reverse r eaction](media/image88.png)
![Solving for \[NOBr21, we have \[NOBQ = CNO\]CBr2\] Substituting this
relationship into Equation 14.28, we have Rate = = where the
experimental rate constant k equals k2k1/k\_1. This expression is
consistent with the experimental rate law (Equation 14.25). Thus, our
alternative mechanism](media/image89.png)

Question 5 (d)
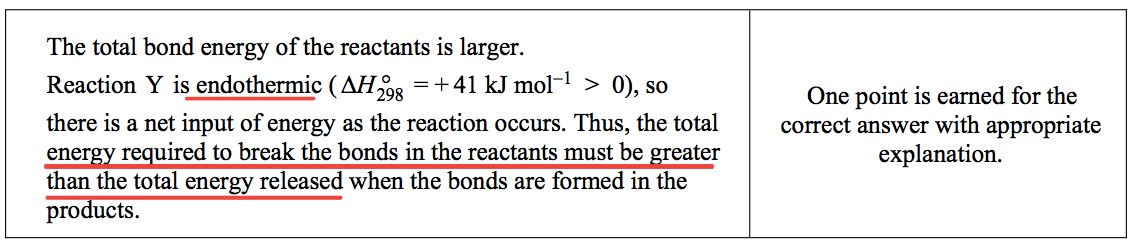
Question 5 (e)
- Thermodynamic data for an overall reaction have no bearing on how slowly or rapidly the reaction occurs
Question 6 (c)
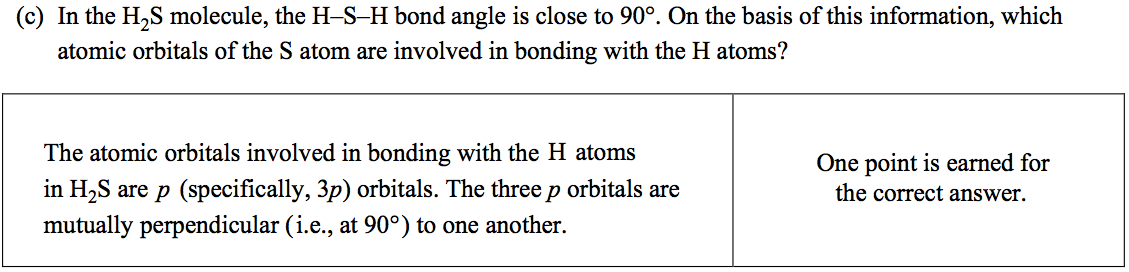
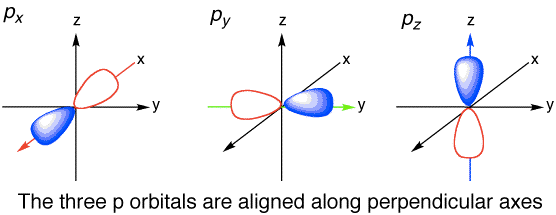
Question 6 (d)
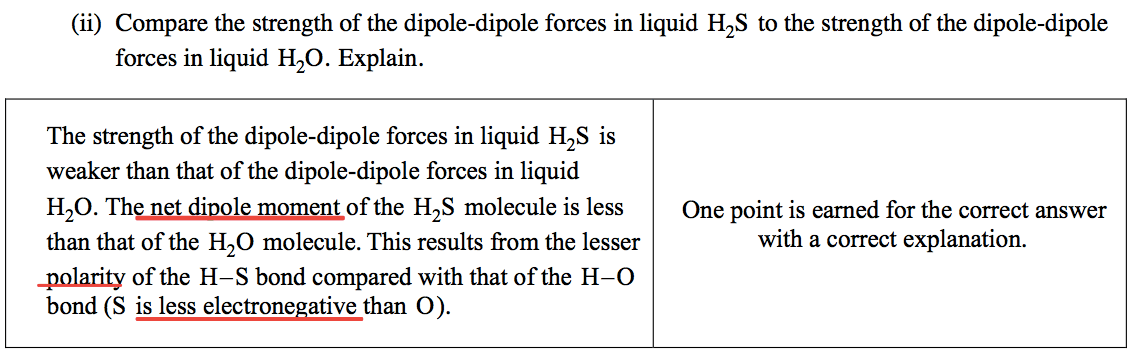
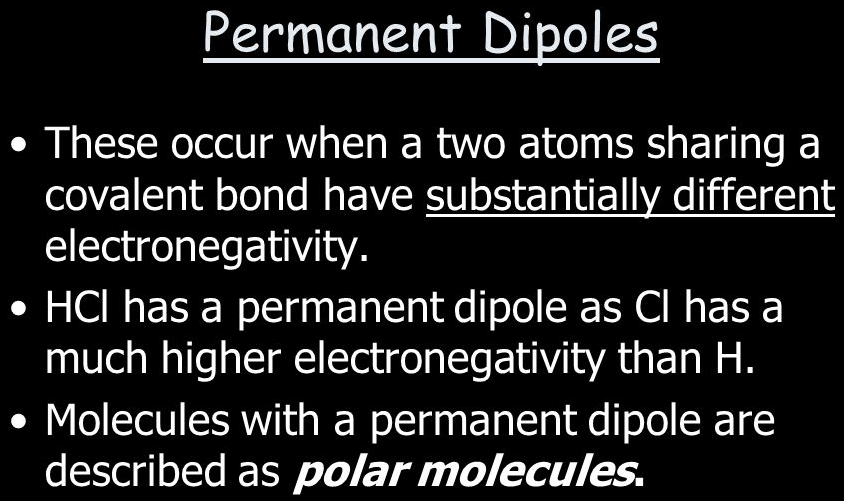
Electronegativity and dipole-dipole force